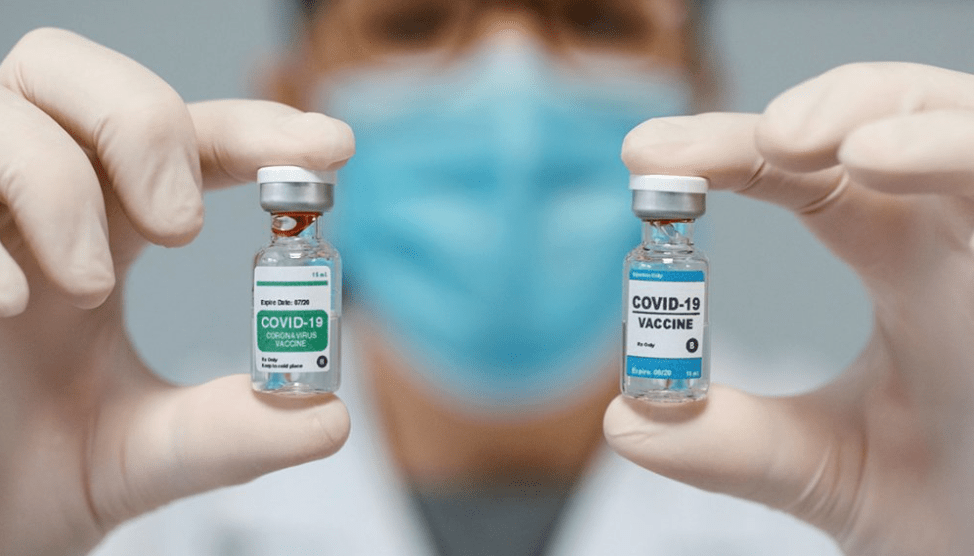
In a joint statement issued today with the CDC, the FDA “recommended a pause in the use of [the J&J vaccine] out of an abundance of caution.” The pause comes amid growing fears over reports that the Johnson and Johnson vaccine may be linked to rare forms of blood clots, much like the AstraZeneca vaccine in Europe.
Each of the six reported cases of blood clots following the vaccine took place in women aged between 18 and 48, with averse symptoms beginning six days after the date of the vaccination. While Johnson & Johnson has yet to find a link between their vaccine and blood clots, they plan to work closely with medical professionals who will be able to determine the actual efficacy of the vaccine. J&J representatives also said that there is little cause for concern if you received their vaccine more than a month ago — symptoms are unlikely to develop after approximately 13 from receiving the vaccine. They also urge those who have received any of the Covid-19 vaccinations to be aware of any unusual symptoms and to contact a medical professional if they are experiencing severe or unknown symptoms.
The CDC and FDA officials will meet on Wednesday to further review the reports of blood clots following the J&J vaccine. While no definitive link has yet been found between the clots and the vaccine, it may be similar to the way that bodies typically respond to other vaccines like the J&J vaccine. Both J&J and AstraZeneca use a virus that causes a version of the common cold as the basis for their vaccinations — this type of vaccination has the potential to cause blood clots in recipients who have a low blood platelet count.
The distrust of the Johnson and Johnson vaccine is proving to be detrimental not only to vaccination efforts but to the value of J&J as a company. J&J shares fell by 3 percent during the day and the pause in the vaccine rollout will likely prevent the company from reaching its goal of administering 100 million vaccines to the United States by the end of May. The company is already behind pace because of manufacturing problems in an Emergent Biosolutions factory that prevented 15 million doses of the vaccine from reaching patients across the United States.
According to the Biden administration’s Covid-19 czar Jeffrey Zients, the pause on the J&J vaccine will not have a significant impact on the goal to vaccinate 200 million people by the end of President Joe Biden’s first 100 days in office. The J&J vaccine only makes up around 5 percent of the total amount of administered vaccines in the United States, and Pfizer and Moderna will likely pick up the slack and increase production of their own respective vaccines in the coming months. THe Biden administration has already secured 300 million doses for the American people, and another 28 million are set to be administered this week.