Cancer is an evolutionary disease. The same forces that turned dinosaurs into birds turn normal cells into cancer: genetic mutations and traits that confer a survival advantage.
Evolution in animals is largely driven by mutations in the DNA of germ cells – the sperm and egg that fuse to form an embryo. These mutations may confer traits that differ from those of the offspring’s parents such as larger paws, sharper teeth or lighter hair color. If the change is beneficial, like a mutation that lightens the hair of a rabbit living in a snowy climate, the animal is better able to survive, mate and pass on its mutated gene to the next generation. Such changes accumulate over millions of years, eventually turning, for example, dinosaurs into bluebirds.
Cancer arises by these same evolutionary pressures, but at the level of individual cells within a person’s body. Instead of animals fighting for survival in a harsh environment, cells compete for space and nutrients. Because different organs are composed of different kinds of cells, cancers arising from different organs differ from one another in appearance and behavior and in how well they respond to treatment.
We are a team of oncologists, pathologists and translational scientists who work together to study how cancers evolve. We believe that understanding evolution is key to understanding how cancer arises and how to treat it.
Human cells are normally in a constant state of death and renewal. Old cells die and are replaced by new ones. These phases of death and renewal are usually orderly, with cells cooperating in a complex process that provides them with proper nutrition and replaces them at a constant rate, maximizing the overall function of the organ they make up.
Mutations disrupt this orderly process. Changes to the cell’s DNA alter the proteins that comprise the cell’s structure and govern its behavior, sometimes in ways that lead it to duplicate itself faster than its neighbors, resist normal death signals and sequester nutrients for itself.
The immune system attacks and kills mutant cells in most cases. However, if one survives and duplicates itself many times over, it can form a tumor made of multiple mutant cells. These tumor cells continue to reproduce and mutate, evolving until the tumor ultimately gains the ability to spread throughout the body.
Cancer detected at the earliest stages of this evolution can be treated more effectively than cancer at more advanced stages. This observation underlies the effectiveness of cancer screening programs in reducing cancer rates.
For example, colon cancer begins as a polyp, a small tumor on the interior surface of the colon that is harmless on its own but may eventually evolve and gain the ability to invade the colon wall and spread throughout the body. Precancerous polyps are easily removed during colonoscopy screenings, preventing them from evolving to invasive colon cancer.
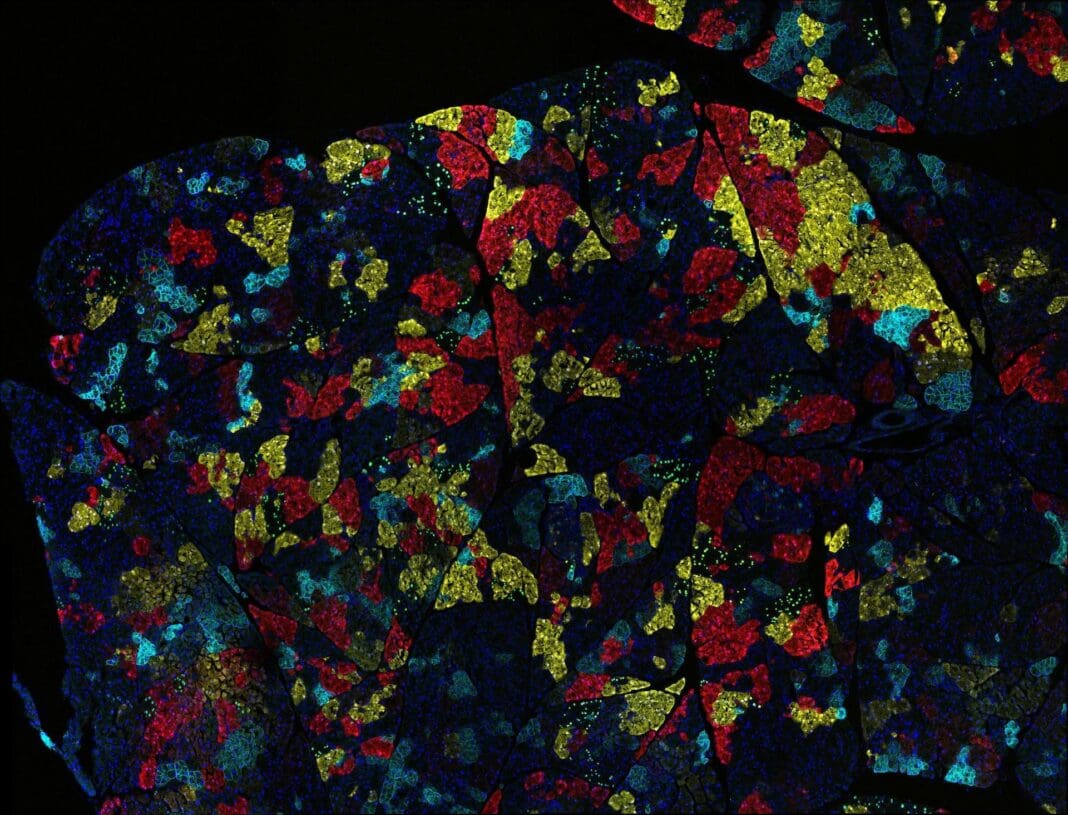
In general, cancers from different organs look distinct from one another and contain different proteins. This leads to variations in how they behave.
Under the microscope, cancer looks like a distorted and disorganized version of the normal tissue from which it arose. Cancer cells tend to contain the same set of proteins as those in healthy organs, and likewise continue to perform many of the same functions. For example, prostate cancer contains large amounts of androgen receptors, proteins that bind to testosterone and drives cells to grow and survive. Androgen receptors both enable normal prostate function and drive growth of prostate cancer.
Tumors arising in a given organ also tend to have mutations in the same set of genes, even among different patients. For example, around half of patients with melanoma, an aggressive type of skin cancer, have a mutation in the BRAF gene that enhances cell growth and survival. In contrast, BRAF mutations are rare in lung cancer.
Cancers also differ in the number of mutations they contain, and this number is strongly associated with the organ from which they arise. The prevalence of mutations is also influenced by mutations in genes that control DNA repair. For example, thyroid cancers typically have a low number of mutations while colon cancers have many mutations, a number that is increased dramatically in tumors that have lost genes involved in DNA repair.
Because of these substantial differences in proteins and mutations, tumors from different organs respond differently to treatment. For example, the majority of patients with testicular cancer can be cured with traditional chemotherapy combined with surgery. However, thyroid cancer and melanoma respond minimally to chemotherapy and require different approaches. Radioactive iodine can only be used to treat thyroid cancer because only thyroid cells take up iodine as part of their usual function.
Tumors that contain a large number of mutations often respond well to immunotherapies that help the patient’s immune system attack cancer cells. This is because the immune system sees tumors with more mutations as more foreign and thus mounts a greater response against them. For example, melanoma and bladder and lung cancers respond well to immunotherapy, particularly those that have lost DNA repair function. In contrast, prostate cancer, which often harbors a low number of mutations, has typically responded poorly to immunotherapies.
Treatment can also push cancer to evolve further, gaining advantageous mutations that help them survive and resist therapy.
For example, a subset of lung cancers is driven by mutation in a gene called EGFR. These are treated with a group of drugs that block the protein the mutant EGFR gene encodes for, slowing the cancer’s growth. Lung cancers treated with these drugs often develop a new EGFR mutation called T790M that confers resistance to most EGFR inhibitors. However, researchers have developed another drug that inhibits proteins with T790M and other EGFR mutations more broadly, improving survival for patients with these types of lung cancers.
Similarly, metastatic prostate cancer is often treated with drugs that block androgen receptors, because it depends on them for growth and survival. Over time, the tumors evolve in response to these drugs and develop mutations that change the androgen receptor, massively increase the amount of androgen receptor they produce or, in some cases, completely change their appearance and protein content so they no longer rely on androgen receptors to survive. In these instances, patients require different therapies to overcome resistance.
The fight against cancer is a fight against evolution, the fundamental process that has driven life on Earth since time immemorial. This is not an easy fight, but medicine has made tremendous progress.
Deaths from cancer in the U.S. have declined since the early 1990s. Much of this is attributable to cancer screening programs and recently developed, more effective drugs. The U.S. Food and Drug Administration approved 332 new drug treatments for cancer between 2009 and 2020. More new drugs are on the way.
This article is republished from The Conversation, an independent nonprofit news site dedicated to sharing ideas from academic experts. The Conversation is trustworthy news from experts, from an independent nonprofit. Try our free newsletters.
Read more: Cancers are in an evolutionary battle with treatments – evolutionary game theory could tip the advantage to medicine Cancer evolution is mathematical – how random processes and epigenetics can explain why tumor cells shape-shift, metastasize and resist treatments
Joshua Warrick receives funding from The National Institutes of Health.
David DeGraff receives funding from the National Institutes of Health, Congressionally Directed Medical Research Fund/Department of Defense, the American Cancer Society, the Bladder Cancer Advocacy Network, and Bristol Myers Squibb.
Monika Joshi receives funding from NIH, AstraZeneca, BMS for research related work. She has received funding for research from Pfizer and Eisai in the past. She has received personal fees for advisory board from Seagen, Sanofi and Bayer in the past.